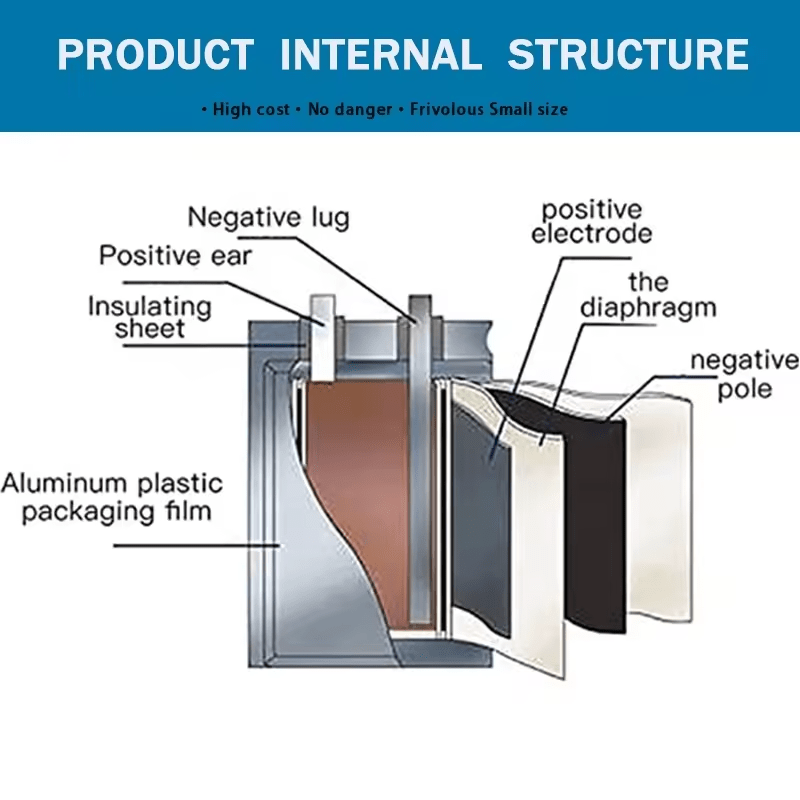
The internal structure of a polymer battery, commonly known as a LiPo battery or lithium polymer battery, is similar to that of a traditional lithium-ion battery, with key differences in the type of electrolyte used. Here’s an overview of the main components:
1. Cathode:
– The cathode is typically made of lithium metal oxides, such as lithium cobalt oxide, lithium manganese oxide, or nickel cobalt manganese oxide, coated onto aluminum foil. This is where lithium ions are intercalated and de-intercalated during battery operation.
2. Anode:
– The anode is usually made of graphite, coated onto copper foil. Lithium ions migrate to the anode during charging and move back to the cathode during discharging.
3. Electrolyte:
– Polymer batteries use a gel-like or solid polymer electrolyte, rather than the liquid electrolyte found in conventional lithium-ion batteries. The electrolyte’s primary function is to facilitate the movement of lithium ions between the cathode and anode while preventing electrons from passing through.
4. Separator:
– The separator is a microporous membrane, usually made of polyethylene or polypropylene, placed between the cathode and anode. Its main role is to prevent short-circuiting within the battery while allowing lithium ions to pass through.
5. Current Collectors:
– The current collectors, made of aluminum foil for the cathode and copper foil for the anode, are responsible for conducting the electric current from the battery’s internal structure to the external circuit.
6. Casing:
– Polymer batteries typically have a flexible aluminum plastic film casing, which allows them to be manufactured in various shapes and sizes, making them ideal for applications requiring design flexibility, such as consumer electronics.
7. Packaging Material:
– The packaging material is part of the casing that encases the entire cell, protecting the internal structure from environmental factors.
Working Principle
During charging, lithium ions move from the cathode through the electrolyte to the anode, where they are intercalated into the graphite layers of the anode. During discharging, the lithium ions move back from the anode to the cathode, while electrons flow from the anode to the cathode through the external circuit, generating an electric current.